重大疾病檢驗
BRCA1/BRCA2 基因檢測
-
BRCA基因突變大大增加普通人群患癌風險
BRCA基因突變大大增加普通人群患癌風險[5][6]
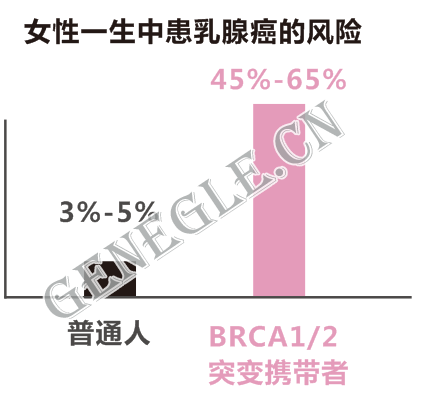
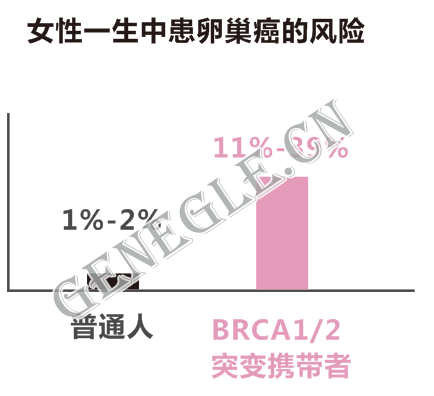
如家族中已有多名成員罹患乳腺癌或卵巢癌,女性BRCA基因突變攜帶者一生中罹患乳腺癌或卵巢癌的風險將提高到85%。
此外,BRCA基因也是靶向治療的靶基因之一。已有研究表明,攜帶BRCA基因突變的卵巢癌患者對鉑類化療較敏感,預后良好,并可獲益于PARP抑制劑(奧拉帕尼)的治療[7]。
-
適合人群及檢測意義
健康人群(*特別是有乳腺癌/卵巢癌家族史者)
評估患乳腺癌/卵巢癌的風險,為疾病早防早治提供參考依據
乳腺癌/卵巢癌患者(*特別是擬使用靶向治療的乳腺癌/卵巢癌患者)
為明確致病原因及制定合理的個性化診療方案提供參考依據
-
檢測方法及檢測內容
檢測方法 PCR擴增+高通量測序
檢測內容 BRCA1(NM_007294.3轉錄本)和BRCA2(NM_000059.3轉錄本)基因的編碼區與鄰近剪接位點
-
檢測優勢及局限性
檢測優勢:
●本檢測覆蓋中國專家共識[8]中建議區域,不僅檢測了BRCA1 (NM_007294.3轉錄本)和 BRCA2(NM_000059.3轉錄本)基因的編碼區域,還覆蓋了鄰近剪接位點,以保證檢測的完整性;
●本檢測采用經過優化的多重PCR擴增體系,擴增均一性好,穩定性高;
●使用美國illumina先進的Hiseq、Miseq等檢測平臺檢測,深度測序分析,有效偵測檢測區域中的低頻突變;
●本檢測中的變異解讀是參照美國醫學遺傳學學會ACMG標準和指南[9],并結合多個權威數據庫進行綜合評價,結 果解讀可靠。
檢測局限性:
●不能檢測大片段的插入、缺失、重復、重排和低頻嵌合現象;
●本檢測覆蓋中國專家共識中包含的區域,但不排除在檢測非覆蓋區存在已/未被發現的致病基因。
-
樣本類型及儲存運輸要求
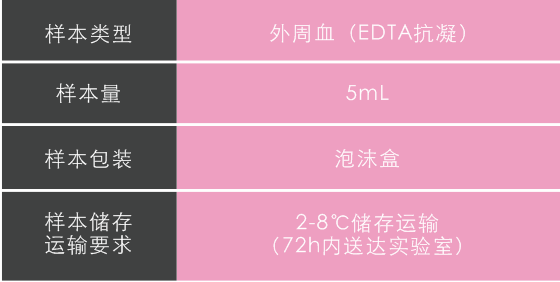
-
檢測流程
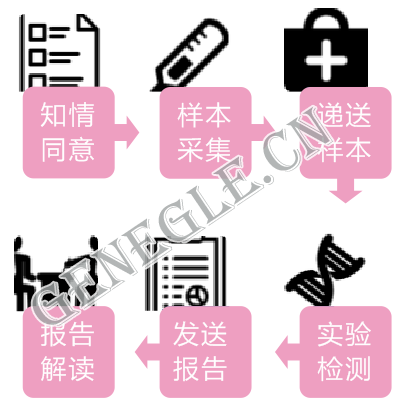
報告周期:13個工作日(樣本送達實驗室后)
注:樣本采集完成后請將樣本連同檢測申請單一起傳送
-
參考文獻
[1]Brose MS, Rebbeck TR, Calzone KA, et al. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. Journal of the National Cancer Institute 2002; 94(18):1365–1372.
[2]Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA 2006; 296(2):185–192.
[3]Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. British Journal of Cancer 2007; 96(1):11–15.
[4]Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. Journal of Clinical Oncology 2009; 27(3):433–438.
[5]Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. American Journal of Human Genetics 2003; 72(5):1117–1130.
[6]Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. Journal of Clinical Oncology 2007; 25(11):1329–1333.
[7]NCCN Guidelines Ovarian Cancer Version 1.2016.
[8]《BRCA數據解讀中國專家共識》, 中華病理學雜志,2017,46(05):293-297.
[9]Richards S (2015). "Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology" Journal of the Association of Genetic Technologists;2015 Third Quarter, Vol. 41 Issue 3, p112














